The manufacturing of medical devices is, appropriately, a closely regulated industry. That’s a good thing. But, the level of scrutiny and high stakes can make changing your manufacturing process feel daunting and risky. You might already know that manual assembly is inefficient, but you also know that the end result is a certified product that meets all regulatory standards. Automating the process might save time and money, but how do you know if you will still be compliant?
The good news is that most of the time, maintaining compliance isn’t very complicated. Starting a new process for a brand-new device is more involved than upgrading an existing process for a device that is already on the market, but regardless of which situation you’re in, you’ve come to the right place. The answers to frequently asked questions (and the links to more information) await.
What is the governing body for medical device manufacturing regulation?
The FDA’s Center for Devices and Radiological Health (CDRH) oversees medical device manufacturing. All of the information in this article, unless otherwise specified, is documented through their website.
Is my product considered a medical device?
Per Section 201(h)(1) of the Food, Drug, and Cosmetic Act, a device is:
An instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
- (A) recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
- (B) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
- (C) intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes. The term “device” does not include software functions excluded pursuant to section 520(o). (FDA, 2022)
If you’re still not sure, this PRESENTATION can help you decide.
Our company is new to medical device manufacturing. How do I register our facility?
If your company is involved in producing medical devices intended for commercial distribution, you must register your company with the FDA. There are specific criteria that determine whether or not your company is required to register, so if you’re not sure, THIS LIST provides a detailed breakdown.
If your company is required to register, you will need to verify your registration information in Q4 every year, and you will need to list the devices you make and the processes associated with them.
The fee for FY 2023 is $6,493, and your registration must be submitted electronically.
If you determine that your company is required to register, this link will help you GET STARTED.
We are already producing our medical device manually. Are we required to report to the FDA if we switch to an automated process?
*Note: This is the only question here not specifically addressed on the FDA’s web site. I reached out directly to their Division of Industry and Consumer Education team, and the following was their response.*
If you make 510(k) exempt or 510(k) devices, you should document the changes and make sure to follow the parts of the quality system (21 CFR 820) that apply to you. If you manufacture Premarket Approval (PMA) approved medical devices, you may need to submit an amendment to all impacted PMAs for the change. If you do manufacture PMA devices, refer to the PMA Supplements and Amendments website to learn more about changes that require FDA’s notification through amendments or supplements.
510(k) DEVICES are devices that require a Premarket Notification (PMN or 510(k)). The Premarket Notification requirement states that any medical device manufacturing company required to register with the FDA must notify the FDA of their intent to market a medical device at least 90 days in advance. This allows time for proper classification of the device.
If your 510(k) device has already been listed, classified, and cleared for distribution, changing from a manual process to an automated one only requires submitting a new premarket notification when the process change is accompanied by a change in function, recommended use, or performance of the product, or a change in materials. In short, simply documenting your process changes is usually sufficient. If you’re still not sure, you’ll find the DETAILED GUIDELINES here.
What is the difference between premarket notification and premarket approval, and how do I know which applies to my device?
There are three device classifications with increased regulatory control from class I to class III. Use this link to DETERMINE HOW TO CLASSIFY YOUR DEVICE.
Any class III device that is new to the market, was introduced to the market after May 28, 1976, or that has been changed or modified in a way that could affect safety or effectiveness requires premarket notification. SUBMIT A 510(k) at least 90 days prior to your intended marketing/ distribution date.
Premarket approval (PMA) is required for class III devices that post a significant risk of illness or injury, OR devices that were not found substantively equivalent to a class I or II predicate through the 510(k) process. PMA requires the submission of clinical data to support your product’s safety and efficacy.
In short, navigating the 510(k) process will clarify whether or not you need to pursue PMA. If your device is found to be substantively equivalent to another approved device, you will likely be required to submit for PMN (510(k)) rather than PMA.
Read up on PREMARKET APPROVAL (PMA) and find links to the PREMARKET APPROVAL DATABASE and the PREMARKET NOTIFICATION DATABASE on the FDA website.
How do I know if our process aligns with current good manufacturing practices (CGMPs)?
It is the manufacturer’s responsibility to define and implement quality systems to ensure their products are consistently meeting specifications. Manufacturers should use PART 820 – QUALITY SYSTEM REGULATION documentation to guide them in establishing their processes. (Additional resources HERE.)
There may be changes to these guidelines on the horizon as the FDA seeks to align more closely with international requirements through PROPOSED RULE 87 FR 10119. Be sure to reference current best practices when considering a process change. (FDA, 2022)
I still have questions, what do I do?
For more information on medical device regulations, visit https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance
If you have questions that aren’t answered here or at FDA.gov, you can reach out to the Division of Industry and Consumer Education team directly at dice@fda.hhs.gov.
Most importantly, don’t let regulatory concerns stand in the way of your company’s evolution. The most intimidating part of navigating the regulatory process is taking the first step. Congratulations, if you’ve read this far, you’ve taken it. When you’re ready to discuss a plan for automating your process, give us a call.
References
U.S. Food and Drug Administration. (2022, September 29). How to Determine if Your Product is a Medical Device. https://www.fda.gov/medical-devices/classify-your-medical-device/how-determine-if-your-product-medical-device#:~:text=To%20determine%20if%20your%20product%20meets%20the%20definition%20of%20a,definition%20of%20a%20medical%20device.
U.S. Food and Drug Administration. (2022, February 23). Quality System (QS) Regulation/Medical Device Good Manufacturing Practices. https://www.fda.gov/medical-devices/postmarket-requirements-devices/quality-system-qs-regulationmedical-device-good-manufacturing-practices
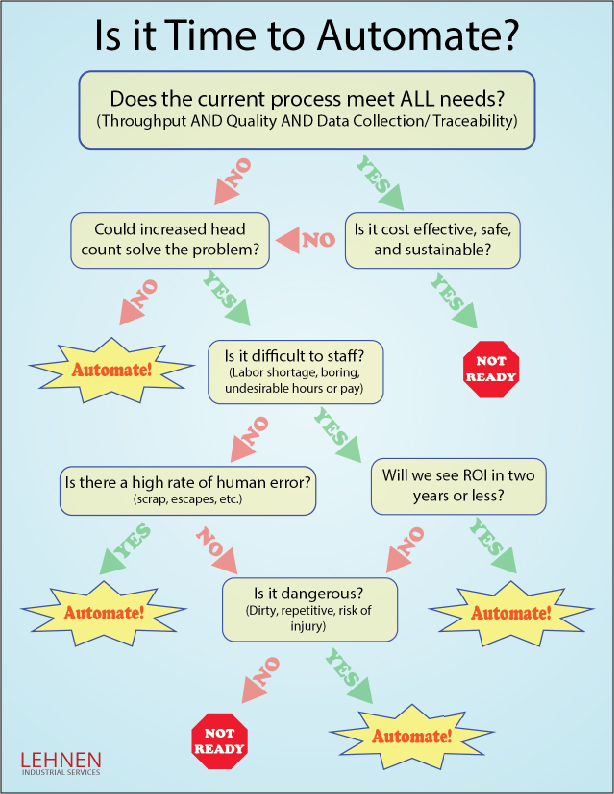
Wondering if it’s time to automate?
Click the image and download this simple decision tree to get started.
Ready for the Next Step?
If you have a process that would benefit from automation, drop us a line.